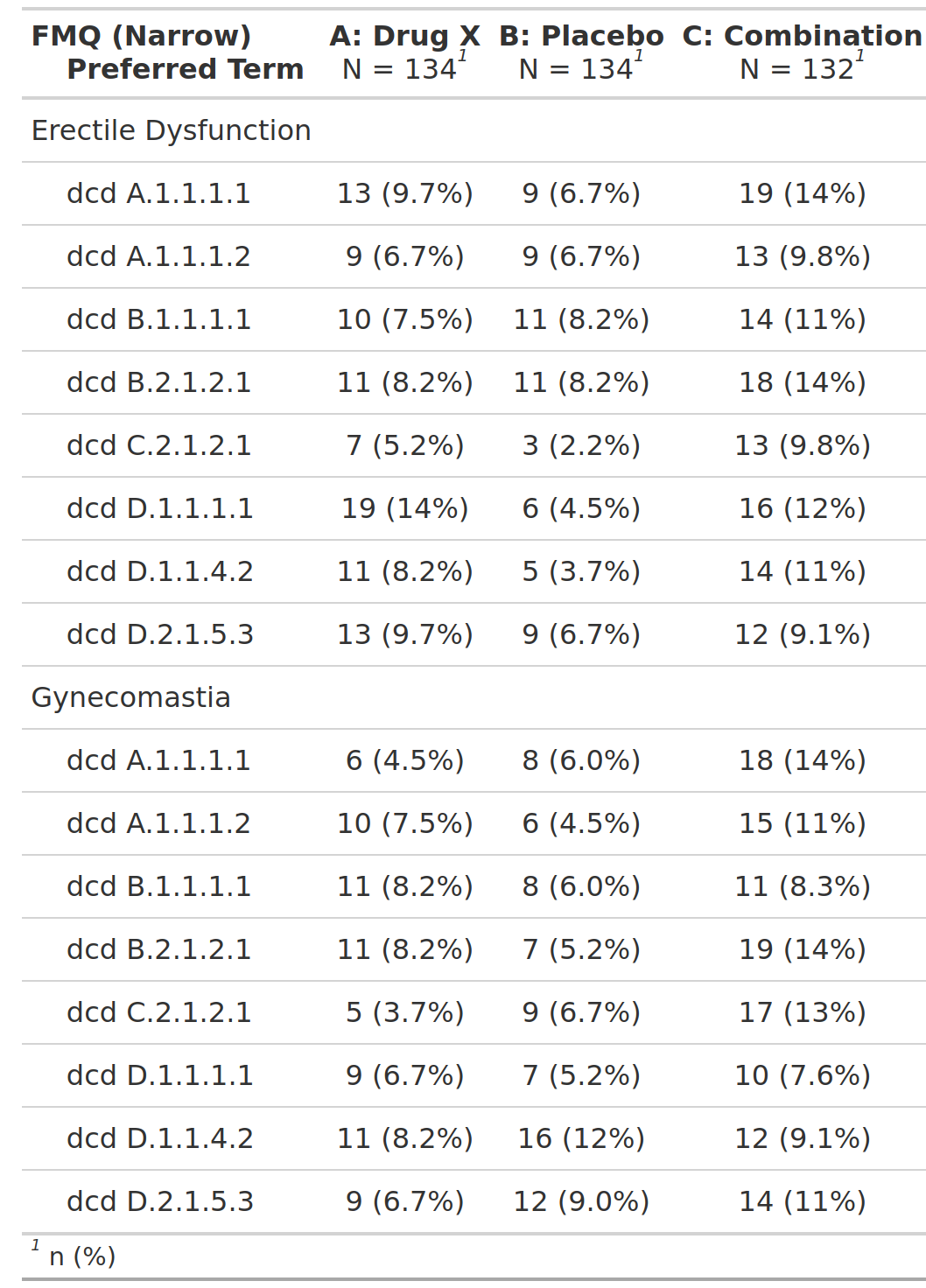
Subjects With Common Adverse Events Occurring at ≥X% Frequency by Preferred Term, Safety Population, Pooled Analysis (or Trial X)
FDA Table 15
table
FDA
safety
adverse events
Code
# Load libraries & data -------------------------------------
library(dplyr)
library(cards)
library(gtsummary)
adsl <- pharmaverseadam::adsl
adae <- pharmaverseadam::adae
# Pre-processing --------------------------------------------
adsl <- adsl |>
filter(SAFFL == "Y") # safety population
data <- adae |>
filter(
# safety population
SAFFL == "Y"
)
$tbl_hierarchical{cards} data frame: 144 x 13 group1 group1_level variable variable_level context stat_name stat_label stat stat_fmt fmt_fun warning error gts_column
1 <NA> TRT01A Placebo tabulate n n 86 86 0 stat_1
2 <NA> TRT01A Placebo tabulate N N 254 254 0 stat_1
3 <NA> TRT01A Placebo tabulate p % 0.339 33.9 <fn> stat_1
4 <NA> TRT01A Xanomeli… tabulate n n 72 72 0 stat_2
5 <NA> TRT01A Xanomeli… tabulate N N 254 254 0 stat_2
6 <NA> TRT01A Xanomeli… tabulate p % 0.283 28.3 <fn> stat_2
7 <NA> TRT01A Xanomeli… tabulate n n 96 96 0 stat_3
8 <NA> TRT01A Xanomeli… tabulate N N 254 254 0 stat_3
9 <NA> TRT01A Xanomeli… tabulate p % 0.378 37.8 <fn> stat_3
10 TRT01A Placebo AEDECOD APPLICAT… hierarch… n n 5 5 <fn> stat_1ℹ 134 more rowsℹ Use `print(n = ...)` to see more rows