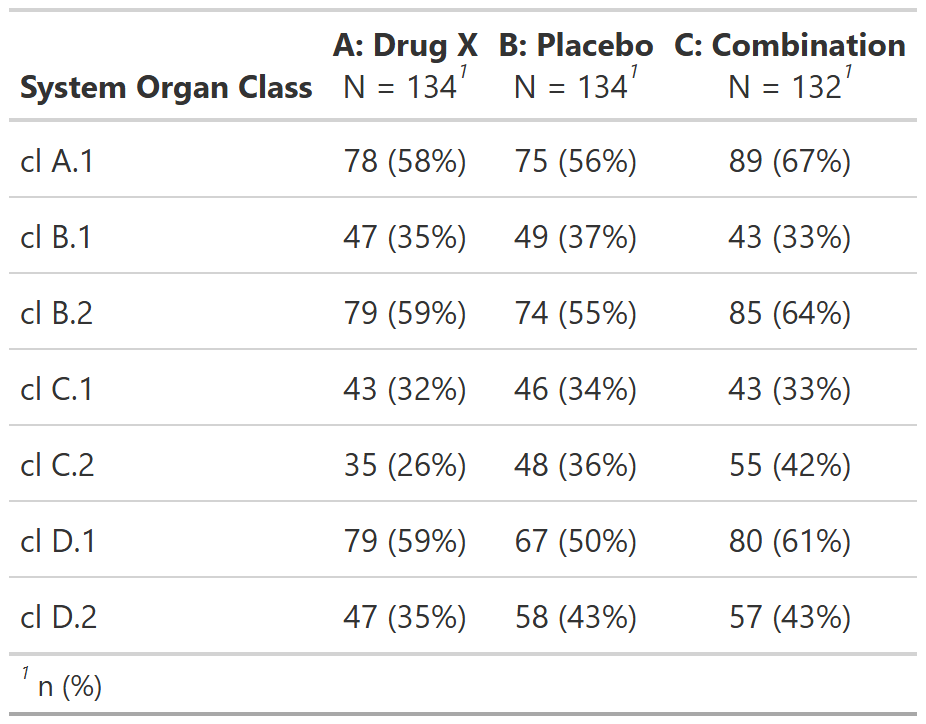
FDA Table 35
Patients With Adverse Events by System Organ Class, Safety Population, Pooled Analysis (or Trial X)
table
FDA
safety
adverse events
Code
# Load libraries & data -------------------------------------
library(dplyr)
library(cards)
library(gtsummary)
adsl <- random.cdisc.data::cadsl
adae <- random.cdisc.data::cadae
# Pre-processing --------------------------------------------
adsl <- adsl %>%
filter(SAFFL == "Y") # safety population
data <- adae |>
filter(SAFFL == "Y") # safety population
{cards} data frame: 72 x 13 group1 group1_level variable variable_level context stat_name stat_label stat stat_fmt fmt_fun warning error gts_column
1 <NA> TRT01A A: Drug X categori… n n 134 134 0 stat_1
2 <NA> TRT01A A: Drug X categori… N N 400 400 0 stat_1
3 <NA> TRT01A A: Drug X categori… p % 0.335 33.5 <fn> stat_1
4 <NA> TRT01A B: Place… categori… n n 134 134 0 stat_2
5 <NA> TRT01A B: Place… categori… N N 400 400 0 stat_2
6 <NA> TRT01A B: Place… categori… p % 0.335 33.5 <fn> stat_2
7 <NA> TRT01A C: Combi… categori… n n 132 132 0 stat_3
8 <NA> TRT01A C: Combi… categori… N N 400 400 0 stat_3
9 <NA> TRT01A C: Combi… categori… p % 0.33 33.0 <fn> stat_3
10 TRT01A A: Drug X AEBODSYS cl A.1 hierarch… n n 78 78 <fn> stat_1
11 TRT01A A: Drug X AEBODSYS cl A.1 hierarch… N N 134 134 <fn> stat_1
12 TRT01A A: Drug X AEBODSYS cl A.1 hierarch… p % 0.582 58 <fn> stat_1
13 TRT01A B: Place… AEBODSYS cl A.1 hierarch… n n 75 75 <fn> stat_2
14 TRT01A B: Place… AEBODSYS cl A.1 hierarch… N N 134 134 <fn> stat_2
15 TRT01A B: Place… AEBODSYS cl A.1 hierarch… p % 0.56 56 <fn> stat_2
16 TRT01A C: Combi… AEBODSYS cl A.1 hierarch… n n 89 89 <fn> stat_3
17 TRT01A C: Combi… AEBODSYS cl A.1 hierarch… N N 132 132 <fn> stat_3
18 TRT01A C: Combi… AEBODSYS cl A.1 hierarch… p % 0.674 67 <fn> stat_3
19 TRT01A A: Drug X AEBODSYS cl B.1 hierarch… n n 47 47 <fn> stat_1
20 TRT01A A: Drug X AEBODSYS cl B.1 hierarch… N N 134 134 <fn> stat_1
21 TRT01A A: Drug X AEBODSYS cl B.1 hierarch… p % 0.351 35 <fn> stat_1
22 TRT01A B: Place… AEBODSYS cl B.1 hierarch… n n 49 49 <fn> stat_2
23 TRT01A B: Place… AEBODSYS cl B.1 hierarch… N N 134 134 <fn> stat_2
24 TRT01A B: Place… AEBODSYS cl B.1 hierarch… p % 0.366 37 <fn> stat_2
25 TRT01A C: Combi… AEBODSYS cl B.1 hierarch… n n 43 43 <fn> stat_3
26 TRT01A C: Combi… AEBODSYS cl B.1 hierarch… N N 132 132 <fn> stat_3
27 TRT01A C: Combi… AEBODSYS cl B.1 hierarch… p % 0.326 33 <fn> stat_3
28 TRT01A A: Drug X AEBODSYS cl B.2 hierarch… n n 79 79 <fn> stat_1
29 TRT01A A: Drug X AEBODSYS cl B.2 hierarch… N N 134 134 <fn> stat_1
30 TRT01A A: Drug X AEBODSYS cl B.2 hierarch… p % 0.59 59 <fn> stat_1
31 TRT01A B: Place… AEBODSYS cl B.2 hierarch… n n 74 74 <fn> stat_2
32 TRT01A B: Place… AEBODSYS cl B.2 hierarch… N N 134 134 <fn> stat_2
33 TRT01A B: Place… AEBODSYS cl B.2 hierarch… p % 0.552 55 <fn> stat_2
34 TRT01A C: Combi… AEBODSYS cl B.2 hierarch… n n 85 85 <fn> stat_3
35 TRT01A C: Combi… AEBODSYS cl B.2 hierarch… N N 132 132 <fn> stat_3
36 TRT01A C: Combi… AEBODSYS cl B.2 hierarch… p % 0.644 64 <fn> stat_3
37 TRT01A A: Drug X AEBODSYS cl C.1 hierarch… n n 43 43 <fn> stat_1
38 TRT01A A: Drug X AEBODSYS cl C.1 hierarch… N N 134 134 <fn> stat_1
39 TRT01A A: Drug X AEBODSYS cl C.1 hierarch… p % 0.321 32 <fn> stat_1
40 TRT01A B: Place… AEBODSYS cl C.1 hierarch… n n 46 46 <fn> stat_2ℹ 32 more rowsℹ Use `print(n = ...)` to see more rows