| ARM | USUBJID | AGESEX | DOSAGE | DOSDUR | DTHADY | DTHCAUS | DTHCAT |
|---|---|---|---|---|---|---|---|
| A: Drug X | AB12345-BRA-1-id-42 | 36/M | 5040 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-BRA-1-id-93 | 34/F | 6720 mg | 37 | 657 | ADVERSE EVENT | ADVERSE EVENT |
| A: Drug X | AB12345-BRA-11-id-345 | 37/F | 7680 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-BRA-11-id-397 | 38/M | 7680 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-BRA-13-id-177 | 24/M | 4800 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-BRA-15-id-36 | 38/F | 7200 mg | 37 | 812 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| A: Drug X | AB12345-BRA-2-id-296 | 44/F | 4800 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CAN-11-id-139 | 31/M | 6000 mg | 37 | 563 | ADVERSE EVENT | ADVERSE EVENT |
| A: Drug X | AB12345-CHN-1-id-123 | 27/F | 5280 mg | 37 | 750 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| A: Drug X | AB12345-CHN-1-id-133 | 25/F | 5520 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-235 | 39/M | 5520 mg | 37 | 968 | ADVERSE EVENT | ADVERSE EVENT |
| A: Drug X | AB12345-CHN-1-id-275 | 27/M | 7200 mg | 38 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-277 | 41/M | 8160 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-315 | 32/M | 6480 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-316 | 25/F | 8400 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-329 | 48/M | 5520 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-47 | 25/F | 5520 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-53 | 48/M | 6960 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-69 | 32/F | 6960 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-1-id-71 | 33/M | 7440 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-116 | 30/M | 8880 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-124 | 41/M | 7440 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-132 | 30/F | 6000 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-153 | 41/M | 7680 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-167 | 28/F | 4800 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-256 | 23/M | 6720 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-354 | 42/F | 5280 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-356 | 28/F | 8160 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-11-id-392 | 39/M | 5280 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-12-id-266 | 32/F | 5520 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-12-id-396 | 25/F | 8880 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-14-id-1 | 27/F | 5760 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-14-id-161 | 33/F | 6480 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-14-id-168 | 39/M | 6960 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-15-id-399 | 33/F | 7200 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-17-id-182 | 37/M | 7680 mg | 37 | 902 | LOST TO FOLLOW UP | OTHER |
| A: Drug X | AB12345-CHN-17-id-309 | 36/F | 7200 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-17-id-92 | 29/M | 5520 mg | 38 | 721 | LOST TO FOLLOW UP | OTHER |
| A: Drug X | AB12345-CHN-18-id-170 | 31/M | 7200 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-2-id-22 | 29/M | 8400 mg | 38 | 409 | MISSING | OTHER |
| A: Drug X | AB12345-CHN-2-id-223 | 29/F | 5760 mg | 37 | 468 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| A: Drug X | AB12345-CHN-2-id-272 | 41/F | 4320 mg | 37 | 773 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| A: Drug X | AB12345-CHN-2-id-274 | 40/F | 5520 mg | 37 | 890 | SUICIDE | OTHER |
| A: Drug X | AB12345-CHN-2-id-284 | 36/F | 7200 mg | 38 | NA | NA | NA |
| A: Drug X | AB12345-CHN-2-id-286 | 30/F | 8400 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-2-id-367 | 40/M | 7440 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-2-id-395 | 28/M | 5520 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-3-id-271 | 24/F | 4320 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-3-id-281 | 41/M | 4560 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-4-id-335 | 33/F | 6720 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-4-id-73 | 24/F | 7920 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-5-id-108 | 28/F | 7440 mg | 37 | 935 | MISSING | OTHER |
| A: Drug X | AB12345-CHN-5-id-273 | 34/F | 6240 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-5-id-348 | 37/M | 5520 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-6-id-30 | 29/M | 5280 mg | 37 | 578 | Post-study reporting of death | OTHER |
| A: Drug X | AB12345-CHN-8-id-205 | 34/F | 5760 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-CHN-9-id-374 | 31/F | 6960 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-GBR-1-id-319 | 36/F | 8160 mg | 37 | 531 | ADVERSE EVENT | ADVERSE EVENT |
| A: Drug X | AB12345-JPN-18-id-189 | 48/M | 7920 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-JPN-5-id-326 | 34/F | 7680 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-NGA-1-id-388 | 39/F | 5760 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-NGA-1-id-46 | 28/M | 6480 mg | 37 | 697 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| A: Drug X | AB12345-NGA-11-id-41 | 29/M | 8160 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-NGA-2-id-353 | 28/M | 6960 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-PAK-1-id-148 | 46/M | 7440 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-PAK-1-id-79 | 37/M | 5040 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-PAK-11-id-187 | 32/F | 6240 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-PAK-12-id-328 | 21/F | 7680 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-PAK-13-id-251 | 32/M | 7680 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-RUS-1-id-52 | 40/F | 6960 mg | 37 | 1069 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| A: Drug X | AB12345-RUS-13-id-70 | 39/F | 6720 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-RUS-6-id-77 | 26/F | 5760 mg | 37 | 495 | ADVERSE EVENT | ADVERSE EVENT |
| A: Drug X | AB12345-USA-11-id-32 | 24/F | 7200 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-USA-11-id-339 | 39/F | 5280 mg | 37 | 878 | UNKNOWN | OTHER |
| A: Drug X | AB12345-USA-3-id-282 | 38/M | 4800 mg | 37 | NA | NA | NA |
| A: Drug X | AB12345-USA-9-id-130 | 40/M | 5520 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-BRA-1-id-236 | 32/M | 6480 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-BRA-1-id-65 | 25/F | 9120 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-BRA-12-id-59 | 36/M | 6480 mg | 38 | NA | NA | NA |
| B: Placebo | AB12345-BRA-4-id-383 | 30/F | 7680 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CAN-1-id-341 | 43/F | 5520 mg | 37 | 1013 | SUICIDE | OTHER |
| B: Placebo | AB12345-CAN-4-id-331 | 34/F | 6480 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-107 | 41/M | 6240 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-156 | 32/F | 6000 mg | 37 | 542 | SUICIDE | OTHER |
| B: Placebo | AB12345-CHN-1-id-186 | 27/M | 5520 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-197 | 31/F | 6960 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-212 | 30/F | 5040 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-33 | 31/F | 6960 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-346 | 21/M | 7920 mg | 37 | 834 | ADVERSE EVENT | ADVERSE EVENT |
| B: Placebo | AB12345-CHN-1-id-400 | 26/F | 6960 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-86 | 31/F | 8640 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-1-id-97 | 39/F | 4080 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-174 | 41/M | 4320 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-220 | 26/F | 4560 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-24 | 46/F | 6720 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-247 | 30/M | 4560 mg | 38 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-278 | 47/F | 6480 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-289 | 40/F | 4800 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-291 | 50/M | 5760 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-317 | 38/F | 6240 mg | 37 | 658 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| B: Placebo | AB12345-CHN-11-id-318 | 42/F | 7920 mg | 37 | 910 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| B: Placebo | AB12345-CHN-11-id-358 | 32/F | 4560 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-362 | 39/F | 6240 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-364 | 48/F | 4800 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-54 | 26/M | 5040 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-11-id-90 | 37/F | 5760 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-12-id-294 | 35/F | 8400 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-12-id-322 | 37/M | 7920 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-13-id-102 | 37/M | 5280 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-13-id-19 | 30/F | 7440 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-13-id-239 | 58/F | 6720 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-13-id-304 | 29/F | 5280 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-14-id-305 | 28/F | 5760 mg | 37 | 406 | Post-study reporting of death | OTHER |
| B: Placebo | AB12345-CHN-16-id-323 | 51/M | 7680 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-17-id-48 | 32/F | 6000 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-2-id-118 | 36/F | 7440 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-2-id-332 | 31/F | 5760 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-2-id-34 | 48/M | 5280 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-3-id-333 | 30/F | 8160 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-4-id-122 | 23/M | 8160 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-5-id-231 | 37/F | 4800 mg | 37 | 468 | ADVERSE EVENT | ADVERSE EVENT |
| B: Placebo | AB12345-CHN-5-id-338 | 37/F | 7920 mg | 38 | NA | NA | NA |
| B: Placebo | AB12345-CHN-6-id-385 | 40/F | 7440 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-7-id-126 | 27/M | 7920 mg | 37 | 1066 | ADVERSE EVENT | ADVERSE EVENT |
| B: Placebo | AB12345-CHN-7-id-202 | 44/F | 9120 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-7-id-267 | 40/M | 5280 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-CHN-9-id-11 | 28/F | 5280 mg | 37 | 398 | UNKNOWN | OTHER |
| B: Placebo | AB12345-CHN-9-id-147 | 26/F | 6240 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-GBR-17-id-211 | 62/M | 5760 mg | 37 | 455 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| B: Placebo | AB12345-GBR-6-id-111 | 30/F | 5520 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-JPN-1-id-21 | 29/F | 7440 mg | 38 | NA | NA | NA |
| B: Placebo | AB12345-JPN-14-id-194 | 28/F | 5040 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-NGA-1-id-325 | 28/F | 5760 mg | 37 | 935 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| B: Placebo | AB12345-NGA-2-id-308 | 26/F | 5520 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-PAK-1-id-112 | 44/M | 4080 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-PAK-1-id-169 | 29/M | 7680 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-PAK-1-id-17 | 33/M | 7680 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-RUS-5-id-29 | 58/M | 6960 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-1-id-138 | 36/M | 8160 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-1-id-261 | 32/F | 4080 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-1-id-269 | 35/F | 8400 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-1-id-394 | 46/F | 6960 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-11-id-157 | 50/M | 6720 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-12-id-152 | 29/F | 6240 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-12-id-226 | 30/M | 5040 mg | 37 | NA | NA | NA |
| B: Placebo | AB12345-USA-4-id-190 | 53/M | 4560 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-BRA-1-id-141 | 35/F | 6960 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-BRA-1-id-265 | 25/M | 6000 mg | 37 | 872 | SUICIDE | OTHER |
| C: Combination | AB12345-BRA-11-id-321 | 33/F | 9360 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-BRA-11-id-9 | 40/M | 7200 mg | 38 | 1091 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| C: Combination | AB12345-BRA-4-id-368 | 46/M | 6720 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-BRA-5-id-234 | 32/M | 4800 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CAN-4-id-324 | 32/M | 6240 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-181 | 47/F | 5520 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-208 | 28/M | 7680 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-233 | 36/F | 7680 mg | 37 | 406 | MISSING | OTHER |
| C: Combination | AB12345-CHN-1-id-279 | 33/M | 7920 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-312 | 34/F | 5280 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-347 | 33/M | 7440 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-376 | 41/F | 5760 mg | 38 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-64 | 34/F | 6960 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-74 | 23/F | 8400 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-1-id-78 | 44/F | 8160 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-11-id-113 | 38/M | 6960 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-11-id-146 | 28/F | 5040 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-11-id-158 | 24/F | 7200 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-11-id-184 | 38/F | 8400 mg | 37 | 1029 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| C: Combination | AB12345-CHN-11-id-192 | 35/F | 8640 mg | 37 | 807 | DISEASE PROGRESSION | PROGRESSIVE DISEASE |
| C: Combination | AB12345-CHN-11-id-2 | 37/F | 8880 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-11-id-298 | 31/M | 6720 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-11-id-63 | 30/M | 6720 mg | 38 | NA | NA | NA |
| C: Combination | AB12345-CHN-14-id-143 | 36/F | 6720 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-14-id-375 | 32/F | 8400 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-14-id-56 | 25/M | 7440 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-15-id-262 | 35/M | 7680 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-15-id-297 | 31/M | 8880 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-16-id-352 | 28/M | 6480 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-16-id-381 | 45/M | 5760 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-17-id-31 | 35/F | 5760 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-17-id-84 | 40/F | 4560 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-18-id-106 | 30/F | 5040 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-2-id-292 | 32/F | 6960 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-2-id-342 | 32/M | 6000 mg | 38 | NA | NA | NA |
| C: Combination | AB12345-CHN-2-id-98 | 48/M | 8400 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-3-id-155 | 35/F | 6000 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-3-id-259 | 27/M | 7440 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-4-id-115 | 38/M | 5760 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-5-id-221 | 40/F | 6720 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-5-id-83 | 44/M | 6960 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-CHN-7-id-290 | 33/M | 6000 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-GBR-11-id-390 | 69/M | 6000 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-JPN-1-id-7 | 54/F | 8880 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-JPN-11-id-135 | 37/F | 5760 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-JPN-11-id-373 | 45/M | 7440 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-JPN-12-id-219 | 37/F | 5520 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-JPN-17-id-393 | 26/M | 7920 mg | 38 | 652 | ADVERSE EVENT | ADVERSE EVENT |
| C: Combination | AB12345-JPN-5-id-252 | 31/F | 4800 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-1-id-198 | 32/F | 7680 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-1-id-357 | 41/M | 5040 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-1-id-43 | 37/M | 6720 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-1-id-96 | 28/M | 5760 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-11-id-173 | 24/F | 6000 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-11-id-334 | 20/M | 5280 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-NGA-11-id-35 | 32/M | 7440 mg | 37 | 923 | ADVERSE EVENT | ADVERSE EVENT |
| C: Combination | AB12345-NGA-4-id-215 | 40/F | 8880 mg | 37 | 647 | ADVERSE EVENT | ADVERSE EVENT |
| C: Combination | AB12345-PAK-1-id-125 | 49/F | 7200 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-PAK-1-id-95 | 28/F | 6240 mg | 37 | 1027 | Post-study reporting of death | OTHER |
| C: Combination | AB12345-PAK-2-id-188 | 23/F | 7200 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-PAK-2-id-191 | 38/F | 5520 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-PAK-5-id-20 | 40/M | 6960 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-RUS-3-id-378 | 30/F | 7440 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-RUS-4-id-6 | 44/F | 4320 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-RUS-7-id-243 | 27/F | 8400 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-1-id-137 | 29/M | 5040 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-1-id-242 | 30/F | 5760 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-1-id-254 | 28/F | 5520 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-1-id-295 | 30/F | 5280 mg | 38 | NA | NA | NA |
| C: Combination | AB12345-USA-12-id-140 | 32/F | 7680 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-19-id-58 | 47/M | 5520 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-2-id-110 | 35/F | 7920 mg | 37 | NA | NA | NA |
| C: Combination | AB12345-USA-8-id-206 | 34/F | 6240 mg | 37 | NA | NA | NA |
FDA Table 8
All Individual Patient Deaths, Safety Population, Pooled Analyses
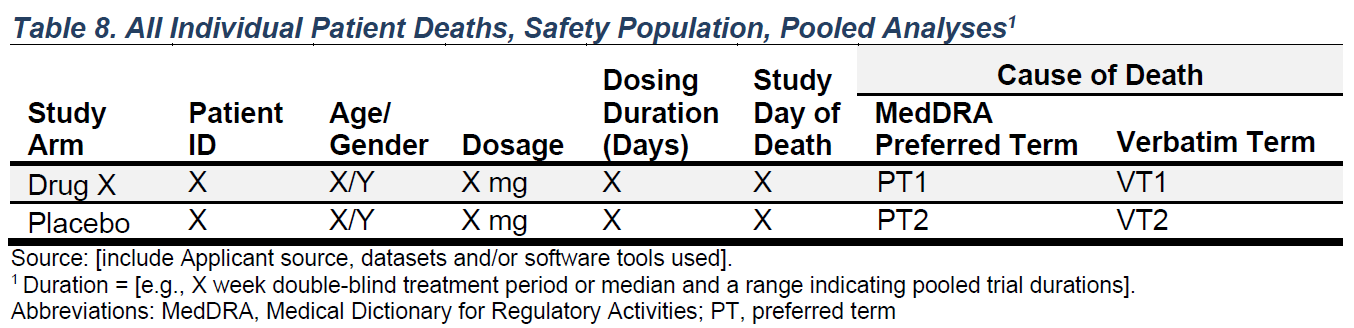
make_table_08()
Required variables:
-
adae:USUBJID,AGE,SEX,AESDTH,DTHADY, and the variables specified bydth_vars,arm_var, andsaffl_var. -
adex(if specified):USUBJID,PARAMCD,TRTSDT,TRTEDT,AVAL,AVALU, and the variable specified bysaffl_var.
| Argument | Description | Default |
|---|---|---|
adae |
(data.frame) Dataset (typically ADAE) required to build table. |
No default |
adex |
(data.frame) Dataset (typically ADEX) required to build table. |
No default |
arm_var |
(character) Arm variable used to split table into columns. |
"ARM" |
saffl_var |
(character) Flag variable used to indicate inclusion in safety population. |
"SAFFL" |
dth_var |
(vector of character) additional death variables from adae to include in the table. |
c("DTHCAUS", "DTHCAT") |
lbl_dth_vars |
(vector of character) labels corresponding to variables in dth_vars to print in the table. Labels should be ordered according to the order of variables in dth_vars. |
c("Cause of Death\nMedDRA\nPreferred Term", "Cause of Death\nVerbatim Term") |
na_level |
(character) String to represent missing values. |
"NA" |
annotations |
(named list of character) List of annotations to add to the table. Valid annotation types are title, subtitles, main_footer, and prov_footer. Each name-value pair should use the annotation type as name and the desired string as value. |
NULL |
Source code for this function is available here.